
Therapeutics have evolved over the period of time. In the modern-day parlance, therapeutics (lines of treatment) have gone “digital”. With increase in frequency of various chronic diseases followed by technological advancements, there is no looking back for the new-fangled course of treatment (the digital one). There are loads of treatments to be glanced through, which follow conventional as well as digital method of treatment.
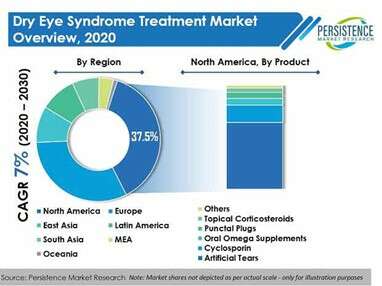
Dry eye syndrome, better known as KCS (keratoconjuctivitis sicca), is amongst the common eye disorder that grips nearly 360 Mn people at the global level. It used to be age-specific initially, but has percolated down to the youngsters as well. Amongst the dry eye syndrome treatments available, CEQUA from Sun Pharmaceuticals Ltd. does make way for cyclosporine, which has been approved by the US FDA.
There is also thyroid eye disease, which causes inflammation of tear glands, eyelids, eye muscles, and fatty tissues behind eyes. At times, double vision may also be the outcome. This condition does affect the thyroid gland and turns out to be chronic in close to half of the patients suffering from eye disorders. Thyroid eye treatment includes corticosteroids, monoclonal antibody, vitamins, and several others. By route of administration, it could be topical and oral.
Coming to diabetic retinopathy, proliferative type does need surgical intervention, as it comes across as one of the advanced stages of diabetic eye disorders. It affects people aged 65 and above. The non-proliferative one affects people aged between 40 and 60. Optical coherence tomography, along with technologically updated devices used for diagnosis, does make way for superlative cross-sectional images that allow the medical practitioners to arrive at better decisions.
As far as eye care products are concerned, based on application, they are for hydrating, for puffiness & dark circles, for eye bags, and sunscreen. Type-wise, they say serum, gel, and cream. These days, online distribution channels are also promulgating the eye care products. Increasing awareness on this part is there for ostentation of eye care products.
Eye care could also be facilitated through eye health products. They are inclusive of omega-3 fatty acids, zeaxanthin and lutein, vitamin A, gamma-linolenic acid, and various others. They help in sustenance of eye function, check the spreading of age-related eye disorders, and lastly – protect eyes from several harmful radiations. Based on form, they could be drops, capsule, and tablet.
Eye care implies eradication of various common health problems like eye allergy, dark circles, puffy eyes, cataract, night blindness, etc. The other factors adversely affecting the eyes include stress and pollution. This calls for sterile eye irrigation solutions. The common end-users include drivers, computer users, and contact lens users. Eye injuries that are caused by radiation, chemical fumes, cuts, and objects could also be cured. They are available, through type of packaging, in the form of squeeze bottle, refill bottle, disposable single use ampules, replacement bottle, and others.
Persistence Market Research (PMR) has, through its detailed study on “Treatments”, has profiled the players as follows:
- Carl Zeiss Meditec AG
- Reckitt Benckiser Group plc
- Alcon
- Fisher Scientific UK Ltd.
- Crest Medical
- Walgreens Co.
- Bausch & Lomb Inc.
- Accutome Inc.
- OPTO-PHARM PTE LTD
- Medline Industries
- Magid Glove & Safety Manufacturing Company LLC
- Sigma Pharmaceuticals, LLC
- Altaire Pharmaceuticals Inc.
- Taumediplast S.r.l
- Nordic Naturals
- Herbalife Nutrition Ltd.
- The Nature’s Bounty Co.
- AMWAY
- Pfizer Inc.
- Allergan Plc
- Glyton
- iS CLINICAL
- PCA Skin
- P&G
- Jan Marini Skin Research
- Abbott
- TheraTears
- Rohoto Group
- Ipsa
- L’Oreal
- Estee Lauder
- Shiseido
- Aerpio Pharmaceuticals
- BCN Peptides
- Kowa Company
- ThromboGenics
- Otysuka Pharmaceutical Co., Ltd.
- Sentiss Pharma Pvt. Ltd.
- OASIS Medical
PMR has also mentioned that Novartis, in April 2019, did receive approval from the US FDA for brolucizumab (an ophthalmic drug). It also says that University of Michigan Kellogg Eye Center is onto evaluation of teprotumumab for treating thyroid eye disease.
PMR has gone ahead to state that North America rules the treatments market, due to extensive scope for research in healthcare. Europe comes in second. PMR has taken a step further in stating that the Asia-Pacific will be the epicentre of experiments, as reduced overheads will help the key players to come up with cost-effective modes of treatment.


